Today marked a major milestone in the fight against Covid-19. A UK grandmother become the first person in the world to be given the Pfizer – BioNTech Covid-19 jab as part of a mass vaccination programme. The Pfizer – BioNTech vaccine is one of three vaccines (the other two being vaccines from Moderna, an American company and Astrazeneca – Oxford University in the UK) that have recently shown promising results in large scale trials. In this post, I’ll describe what I learnt about these vaccines from a couple of recent articles in the Economist, probably my favorite magazine at this point.
I’ll start off with a joke (also from the Economist). Waiting for a breakthrough in the fight against Covid-19 has been a bit like waiting for a bus to arrive. After almost a year of watch-checking and neck-craning, three come along at once :-).
The table below shows the operating mechanism, administration, storage and effectiveness stats for the three vaccines. Incidentally, making tables such as these is not easy in WordPress. I resorted to using Google slides to make the table and then exporting the sheet as a PDF. I then took a screenshot of the PDF and attached the image to the post. Phew!
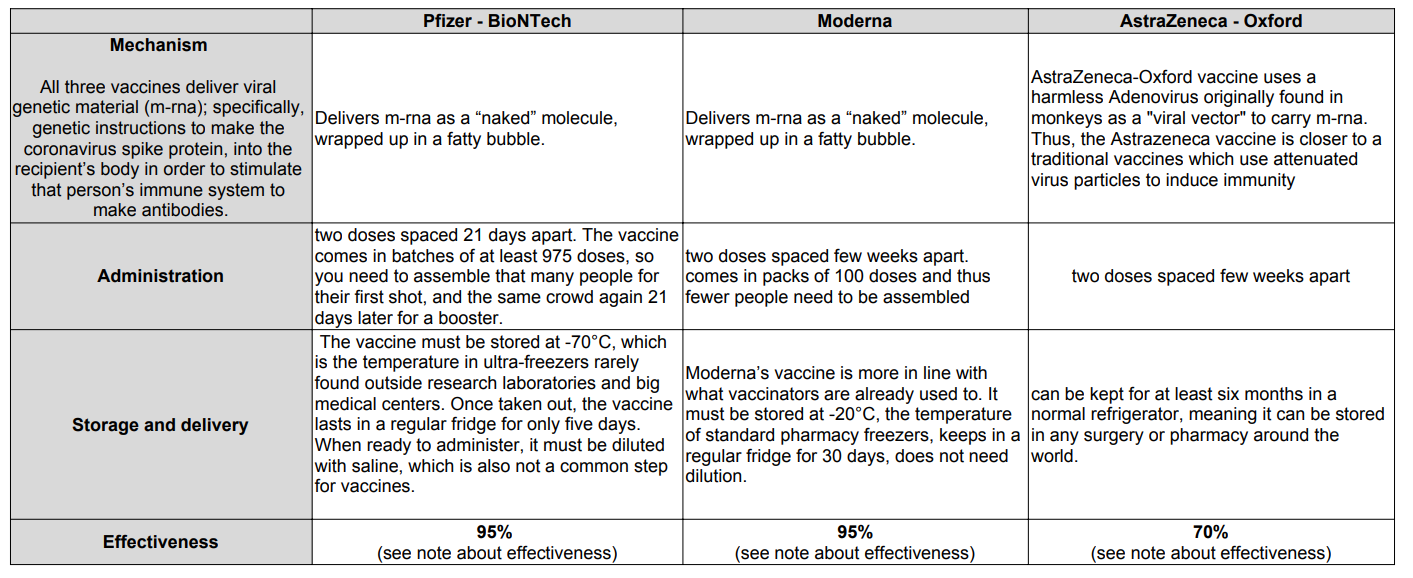
Note about effectiveness:
- The claim of 70% efficacy for the Astrazeneca – Oxford vaccine is complicated by concerns about a slip-up during the trials which meant some participants were given only a half-dose of the vaccine to start with, followed by a full one. Intriguingly, the efficacy of this approach seemed to be about 90%. But if that is confirmed as correct, it follows that the efficacy of the intended protocol is actually only 62%.
There is an intuitively satisfying explanation for the surprisingly higher efficacy of the half-dose regimen. Some researchers had worried that going down the viral-vector route might cause people to develop immunity to the vector as well as to the coronavirus proteins which the m-rna payload would encourage that person’s cells to make. The findings from the accidental subgroup hint that anti-vector immunity may, indeed, be happening, causing a large first dose to prime the body to develop antibodies to the vector. These would then attack the second-dose vectors before they could do their job. A smaller first dose might diminish this priming effect—explaining the better efficacy of unequal doses. - The headline figure of 70% efficacy for the Astrazeneca – Oxford vaccine contrasts superficially with announced values of 95% for the other two vaccines. But, tempting as it is to compare these figures, that is actually hard to do. The most important reason for this is that developers count covid-19 cases in different ways. In particular, the AstraZeneca-Oxford trials checked participants for asymptomatic infection, rather than relying on self-reported symptoms to establish who was infected. The number of cases after vaccination may therefore seem higher than for the Pfizer-BioNTech and Moderna jabs, which relied on self-reporting by patients with a follow-up confirmatory test.
As you can see, the three vaccines offer interesting trade-offs. The Moderna and Pfizer vaccines appear more effective but must be distributed at much lower temperatures, making it more difficult and expensive to scale distribution supply chains, particularly in poorer countries. Astrazeneca – Oxford vaccine is easier to distribute, but appears less effective, although more data needs to be gathered to be sure. Astrazeneca has also pledged to distribute its vaccine to the world at cost.
Dec 15, 2020: According to a WSJ article, The Food and Drug Administration said Tuesday that the Covid-19 vaccine developed by Moderna Inc. MRNA -1.19% is “highly effective,” suggesting the vaccine could soon be added to the arsenal against the pandemic. The next step is for the “Vaccines and Related Biological Products Advisory Committee Meeting” to review the data and issue a recommendation, followed by the final emergency approval from the FDA, which can happen later this week.
The document presented by Moderna is here
Some interesting points:
The study included 27,817 participants randomized 1:1 to vaccine or placebo with a median 7 weeks of follow-up post-dose 2 were included in the per-protocol efficacy analysis population of participants without evidence of SARS-CoV-2 infection prior to vaccination. In other words, vaccine participants didn’t have Covid before the study. Efficacy in preventing confirmed COVID-19 occurring at least 14 days after the second dose of vaccine was 94.5.0% (95% CI 86.5%, 97.8%) with 5 COVID-19 cases in the vaccine group and 90 COVID-19 cases in the placebo group. Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic group.
Study participants were followed up after the study. People who reported having Covid symptoms were given an unscheduled test. A confirmed Covid-19 case was defined as:
- At least TWO of the following systemic symptoms: Fever (≥38ºC), chills, myalgia, headache, sore throat, new olfactory and taste disorder(s), OR
- At least ONE of the following respiratory signs/ symptoms: cough, shortness of breath or difficulty breathing, OR clinical or radiographical evidence of pneumonia; AND
- NP swab, nasal swab, or saliva sample (or respiratory sample, if hospitalized) positive
The study doesn’t draw conclusions about:
- Asymptomatic infections: The study doesn’t include people who may have been infected but not showing symptoms
- Vaccine effectiveness against long-term effects of COVID-19 disease
- Vaccine effectiveness against transmission of SARS-CoV-2: Data are limited to assess the effect of the vaccine against transmission of SARS-CoV-2 from individuals who are infected despite vaccination. Demonstrated high efficacy against symptomatic COVID-19 may translate to overall prevention of transmission in populations with high enough vaccine uptake, though it is possible that if efficacy against asymptomatic infection were lower than efficacy against symptomatic infection, asymptomatic cases in combination with reduced mask-wearing and social distancing could result in significant continued transmission.
Another document published by the FDA (this wasn’t easy to find), provides some evidence that the moderna vaccine is effective in preventing asymptomatic infections. According to the document: Amongst baseline negative participants, 14 in the vaccine group and 38 in the placebo group had evidence of SARS-CoV-2 infection at the second dose without evidence of COVID-19 symptoms. Thus were approximately 2/3 fewer swabs that were positive in the vaccine group as compared to the placebo group at the pre-dose 2 timepoint, suggesting that some asymptomatic infections start to be prevented after the first dose.
They must have taken swabs for study participants before the first and second dose, and that’s how they have this data..
Moderna expects having approximately 20 million doses available in the U.S. by the end of 2020. Additionally, the Company expects to have between 100 million and 125 million doses available globally in the first quarter of 2021, with 85-100 million of those available in the U.S. and 15-25 million of those available outside of the U.S.
I’ll end with a heartening note. The Pfizer – BioNTech vaccine celebrates the potency of science as a global endeavor. In the development of this vaccine, BioNTech, a small German firm founded by a first-generation Turkish couple successfully worked with an American multinational company (Pfizer) headed by a Greek chief executive 🙂
Let’s raise a glass to science, our healthcare workers, medical researchers and scientists and life returning back to normal next year!

Leave a Reply